Early detection of potential drug-induced liver injury due to mitochondrial damage and elucidation of the underlying mechanisms
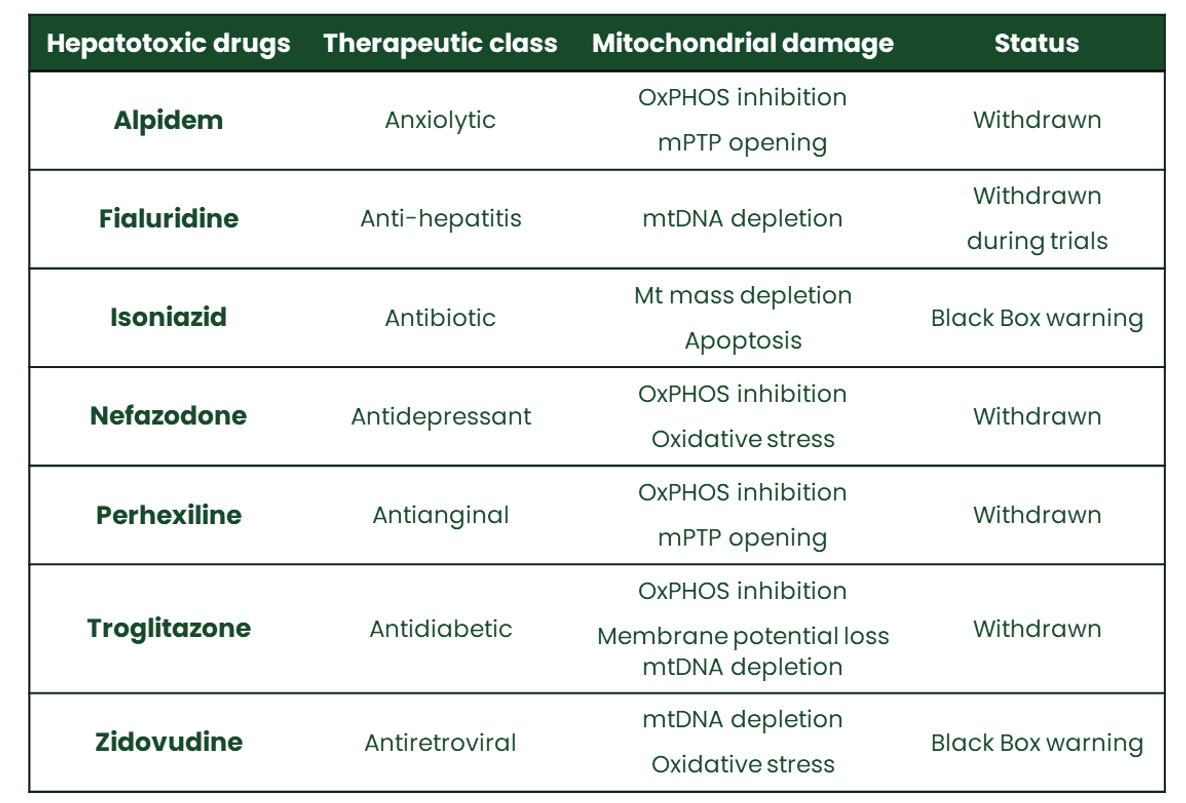
Hepatotoxicity induced by drugs or other xenobiotics – known as drug-induced liver injury, DILI – represents a major health issue. In the clinic, hepatotoxicity has been observed with all therapeutic drug classes.
Investigation of mitochondrial toxicity early during the drug discovery process is thus recommended to eliminate drugs with an unfavourable safety profile. Extensive reference compounds testing by Mitologics has demonstrated that ex vivo assays using isolated mouse liver mitochondria are highly predictive of human drug-induced hepatotoxicity [Porceddu et al., Tox Sci 2012; Buron et al., Env Toxicol 2016].
Tests run on MiToxView® platform are designed to allow early detection of hepatotoxicity due to mitochondrial damage.
Drug-induced mitochondrial dysfunction plays a major part in liver injury due to drastic metabolic perturbations and cell death. The discovery of such toxicity mechanisms leads to discontinuation of clinical trials, boxed warning, or even withdrawal of previously approved drugs.
Through its MiToxView® platform, Mitologics offers a competitive range of highly predictive tests on differentiated hepatic cells (HepaRG®) and liver mitochondria making early detection of hepatotoxicity risks due to mitochondrial damage.
Our assays help to identify acute and long-term mitochondriotoxic drugs or metabolites suspected of inducing hepatotoxicity, while also shedding light on the underlying mechanisms. Indeed, MiToxView® can be used to characterise drug-induced mitochondrial dysfunction and its harmful consequences thanks to its capacity to assess: