Delve deeper into how your compounds modulate energetic metabolism
Mitochondria are key players in the cell’s metabolism, providing a large proportion of its energy, in parallel with glycolysis. Mitologics’ technological platforms MitoXpert® and MitoPathway®, provide robust, custom-designed assays to investigate energy metabolism and the associated mitochondrial machinery.
Mitochondria generate energy – in the form of ATP – by oxidative phosphorylation. They can use many substrates: pyruvate from glycolysis, fatty acids, glutamine, and redox cofactors.
Multiple diseases – such as liver failure and cardiovascular or neurodegenerative diseases – are associated with metabolic imbalances due to impairment of the respiratory chain. In cancer cells, energy metabolism is generally tipped in favour of glycolysis – known as the Warburg Effect – or on the contrary sustained by extremely high oxidative phosphorylation activity.
Detect protective properties of compound in pathological contexts such as respiration impairment in Ischemia-Reperfusion injury.
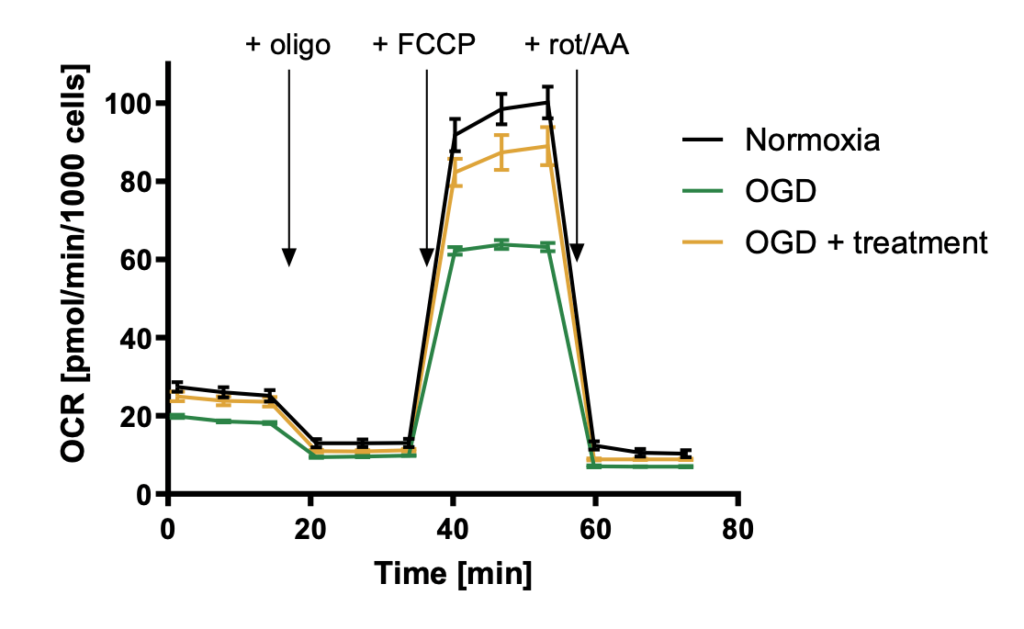
Reduction of oxygen consumption rate (OCR) during reperfusion in oxygen-glucose deprivation (OGD) model and recovery by compound treatment. In this example, the LLC-PK1 cells were exposed to 1% O2 in absence of glucose and back to normoxia before OCR measurement with Seahorse XFe96.
Evaluate compound’s effect on respiratory chain and glycolysis contribution to ATP production.
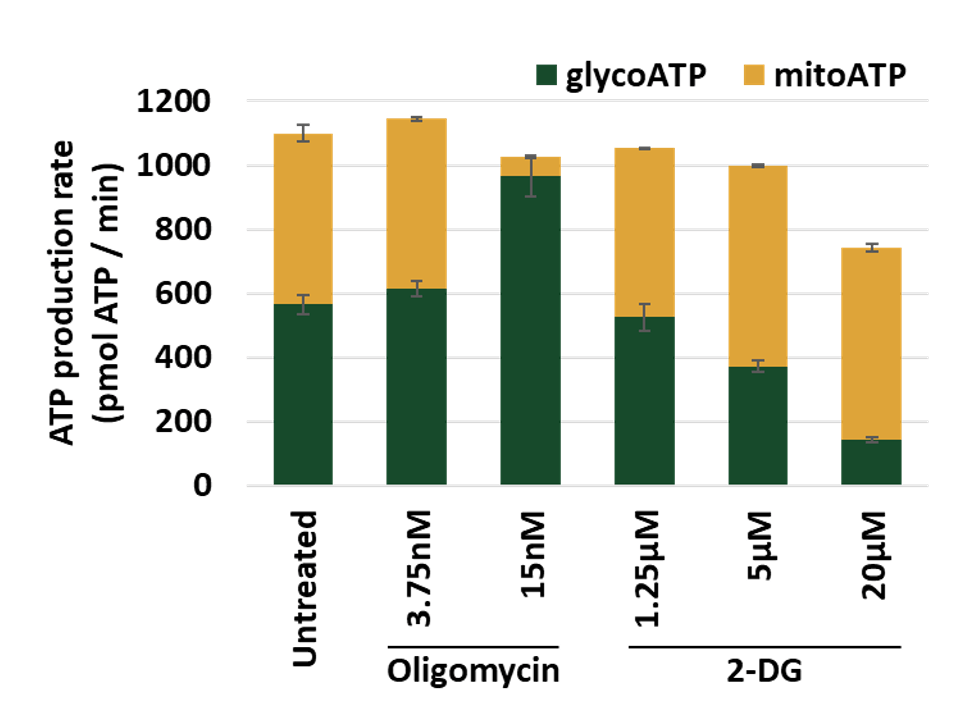
Measurement of the metabolic shift of Hap1 cell line exposed to oligomycin (OxPHOS inhibitor) or 2-deoxyglucose (glycolytic inhibitor). The proportion of ATP produced by respiratory chain (mitoATP) and ATP coming from glycolysis (glycoATP) is estimated with Seahorse XFe96.
Our technological platforms MitoXpert® and MitoPathway® offer assays focusing on energy metabolism and the mitochondrial OxPHOS machinery. We provide consolidated data that can help to:
- Evaluate the efficacy of compounds or actives on critical mitochondrial functions in whole cells, measuring oxygen consumption (OCR), glycolysis (ECAR), and using Seahorse XFe-96 to estimate their respective contributions to ATP production,
- Investigate the direct effects of compounds on mitochondrial respiratory chain functionality: oxygen consumption driven by complex I, complex II, or beta-oxidation of fatty acids (mtFAO); respiratory chain complex activities,
- Identify potential targets of compounds among different mitochondrial components, such as respiratory chain complexes,
- Detect compounds with protective properties in a pathological context: metabolic and oxidative stress, such as nutrient imbalances, hypoxia, oxygen-glucose deprivation…,
- Validate compounds’ capacity to restore mitochondrial function in cells from patients with mitochondrial deficiency,
- Explore the impact of therapeutics – small molecules, antibodies… – on tumour cell metabolism as mitochondria are a key component of the Warburg effect that confers chemotherapy resistance,
- Identify specific respiratory chain complexes targeted by candidate anti-tumour compounds,
- Characterise the metabolic profiles of T cells as part of the new metabolic reprogramming approach to improving the ability of T cells to withstand metabolic stress and tumour microenvironment, ultimately leading to better efficacy of adaptive T cell therapies.