About
Our compagny
Ambitious molecules deserve powerful assays
Our mission is to help develop safe and efficient molecules of benefit to patients. We track and analyse if and how compounds affect mitochondrial functionality and integrity.Our services are designed for scientists across the board, from the pharmaceutical, biotech, cosmetic, chemistry and agrochemistry sectors, to academia.

Mitologics SAS – created in 2009 – is based in the Paris region. The founding team consists of two women scientists – Dr Annie Borgne-Sanchez and Dr Nelly Buron – and Mathieu Porceddu. We consider it a shared vision stemming from our combined experience in academic research, the pharmaceutical industry and biotechnology company.
Using our scientific and technical backgrounds in cellular and molecular biology, we have developed and validated affordable in vitro and ex vivo mitochondrial assays to assess the toxicology and efficacy of test compounds. Today, our approach has been honed through experience gathered over the years working with more than one hundred clients based mainly in Europe, the USA, and Asia.
To provide our customers with the best possible results, we strive for reliable operation, always on the lookout for the optimal experimental setting to meet clients’ needs.
Management team

Dr. Annie Borgne-Sanchez

Dr. Nelly Buron

Mathieu Porceddu

Dr. Cécile Martel

Claire Pertuiset

Léa Zennaro
History
An international player in the mitochondria field since 2009

IMI-Respiratory NTM project– Progress novel assets for non-tubercular mycobacteria (NTM) causing lung diseases – awarded funding from the European Union and Johnson & Johnson. The project coordinated by Dr Meidert H. Lamers (Leiden University, Netherlands) is run in collaboration with Janssen Pharmaceutica NV (Belgium).

IMI-Respiratory TB project– New treatments for Tuberculosis (TB) – awarded funding from the European Union and Johnson & Johnson. The project coordinated by Dr Meidert H. Lamers (Leiden University, Netherlands) is run in collaboration with Janssen Pharmaceutica NV (Belgium).

IACANT project – New compounds targeting mitochondria in cancer cells – gained support from the Paris-Région – as part of its Investissement d’Avenir PIA3 programme. The project was steered by Mitologics.

In January, Mitologics listed as a supplier on Scientist.com, the world's largest marketplace for research services.

The MITOXDRUGS project, to study and predict drug-induced steatosis, awarded funding by the French national research agency (ANR-CE) as part of their “Vie Santé et Bien-être” call. Dr. Bernard Fromenty (Inserm U1241, Université de Rennes 1) was PI for this project.

Mitologics established a partnership with Toxys (Leiden, Pays-Bas) to combine MiToxView® and Tox Tracker® platforms to better predict the risks of compound toxicity.
Find out more > Press release, March 2016

Mitologics contributed to the CARMMA programme – CARdiac and skeletal Muscle alteration in relation to Metabolic diseases and Ageing: role of Adipose Tissue – supported by Hospital-University Research in the health sciences (RHU en santé) through its Investissement d’Avenir programme. The project PI was Prof. Geneviève Derumeaux (Hôpital Henry Mondor, Créteil).

Mitologics became a member of AFSSI, the French association of R&D service providers contributing to innovative research programmes in Life Sciences.

Award to Mitologics from the French Ministry of Education and Research during its National competition for assistance with the creation of innovative technology companies (Concours national d’aide à la création d’entreprises de technologies innovantes) in the Création & Développement category.

Mitologics reaches a Crédit-Impôt-Recherche (CIR) agreement for R&D contract services.

Mitologics recognized in the competition for new talent (Concours des talents) run by the Paris Region.

Mitologics contributed to the PHARMECO project on hepatotoxicity of contaminants in medicines. The project was funded by the French national research agency (ANR-CES), in the “Contaminants, Ecosystems, Health” category; PI Dr. Bernard Fromenty (INSERM U1241, Université de Rennes 1).

Mitologics established a partnership with Horizon Discovery Ltd (Cambridge, UK), including a joint marketing agreement for the use of X-ManTM cell lines, bearing mutations in P53, Ras and PI3K.

Mitologics contributed to the URAAPO project on ADP/ATP translocator, awarded funding from the French national research agency (ANR-PCV); PI Dr. Catherine Brenner (Inserm U769, Châtenay-Malabry).

Mitologics established its laboratory at Robert Debré Hospital (Paris, France).

Award to Mitologics from the French Ministry of Education and Research at the National competition for assistance with the creation of innovative technology companies (Concours national d’aide à la création d’entreprises de technologies innovantes) in the Emergence

Mitologics SAS created in February.
Why focus on mitochondria?
Mitochondria: an essential organelle and a valuable therapeutic target
Mitochondria control the balance between life and death in eukaryotic cells, playing a key role in drug development. As they may be harmed by many compounds, examining mitochondrial function is an important checkpoint for compound safety.
In addition, this organelle constitutes a target of choice to develop innovative medicines for a variety of diseases, or anti-ageing compounds aiming to prevent age-related damage. Mitologics’ offer meets these two needs, by providing services to test both toxicity and efficacy.
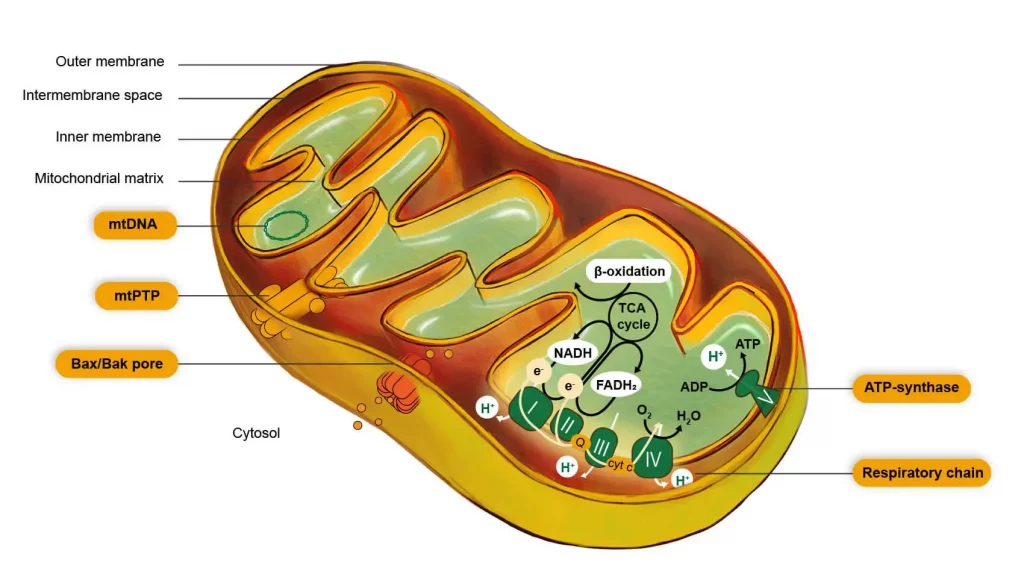
Mitochondria form a highly dynamic network of specialized compartments: the outer membrane, the intermembrane space, the inner membrane and its cristae, and the matrix containing the mtDNA. These sites work together to create the optimal conditions for metabolic reactions.
As the cell’s energy factory, mitochondria play a major role in ATP production by the respiratory chain, with the main enzymes located in and around the inner membrane. The OxPHOS machinery catalyses a chain reaction transforming oxygen and substrates coming from fuel molecules (glucose, glycogen, fatty acids, etc.) into CO2 and H2O, producing ATP. Mitochondria are thus an essential organelle in all eukaryotic cells.
The mitochondrion contributes significantly to cellular oxidative stress, as a result of electron leaks from the respiratory chain – mainly at complex I and complex III – leading to the production of reactive oxygen species (ROS). The mitochondrion is itself a target of ROS, with effects including irreversible damage to mtDNA, mitochondrial membrane lipids, and mitochondrial proteins, resulting in mitochondrial dysfunction, and potentially creating a vicious cycle. To protect itself, the mitochondrion contains an efficient antioxidant system associated with repair system maintaining ROS at low level under non-pathological conditions.
In addition to its role in energy metabolism, one of the mitochondrion’s key roles is to control cell death. Death-regulating proteins located in the outer membrane, make the membrane permeable when triggered by death stimuli. As a result, pro-apoptotic molecules are released from the mitochondria, leading to a cascade of death signals.
Finally, mitochondrial dynamics is part of the cell’s quality control system, guaranteeing a continual pool of functional mitochondria. The processes involved include mitochondrial biogenesis, fusion and fission, to maintain mitochondrial function, as well as mitophagy to eliminate damaged mitochondria.
Working with us: how, when, why
Bespoke services to boost industrial and academic sectors’ projects, from compound discovery to commercialization, compliant with the 3R principle

Since 2009, we have used our expertise and know-how in the field of mitochondria to design and propose studies meeting our clients’ needs.
Mitologics operates for the biotech/pharmaceutical, cosmetic, chemistry and agrochemistry sectors as well as academic laboratories. Following discussions with clients’ CSO, R&D director, project manager, head of project, or scientists, we can provide:
- Fee-for-service R&D studies,
- Consulting services,
- Collaborative public-private projects.
Mitologics intervenes at different stages of clients’ projects:
Drug discovery & Preclinical stage
Potential mitotoxicity can be verified at each stage of drug discovery, in high- or medium-throughput assays. Compounds that present signs of organ toxicity in preclinical in vivo models should be tested on our MiToxView® platform. Results can help determine if and how mitochondrial dysfunction causes organ toxicity, allowing you to adapt the drug development strategy.
Mitologics offers two other platforms: MitoPathway® and MitoXpert®. Derived from relevant in vitro models, results can demonstrate efficacy and allow exploration of the mechanism of action (MoA) of compounds and/or the involvement of mitochondria in particular diseases.
Clinical phases
Mitologics can investigate the impact of a given compound on mitochondrial function/content and cell death pathways in human blood samples (PBMCs) or biopsies collected during clinical trials. These tests are run on our MiToxView® and MitoXpert® platforms.
Commercialization
Customized bio-assays can be set up and validated by Mitologics during drug development. These assays can then be routinely used in a GMP-compliant environment for the release of commercial batches.
Our tests represent an alternative to animal testing, helping to make your studies compliant with the 3R principles.
Replacement, reduction, refinement: Mitologics’ approach can be a fully operational alternative to animal testing.
Investigations are undertaken using cultured human cells or mitochondria isolated from human cell lines, as part of the Replacement principle.
Ex vivo assays on mitochondria purified from rodent organs allow numerous compounds to be tested at a range of doses. This process allows a 40-fold reduction in the number of rodents required for preclinical studies. MiToxView® thus allows you to integrate Reduction in your practice, without risking the quality of your results.
Finally, MiToxView® can robustly identify mitotoxic compounds at an early stage, thus reducing the number of in vivo studies required, or helping to guide further development, meeting both the Reduction and Refinement criteria.
All discussions and exchanges with customers or partners are confidential whether an NDA is signed or not. The intellectual property in relation to any projects or products we receive is strictly respected, as mentioned in our contracts.
The experimental phase starts as soon as the study proposal has been signed, and the compounds or biological materials have arrived at our lab facilities at the Faculté de Santé de Créteil (France).
Testimonials

Careers at Mitologics
Mitologics is open for applications from talented and skilled collaborators.
If you are interested in joining our team, please contact us.